|
| We had a fantastic time at this year’s SOT live meeting, reuniting with all our colleagues in the area of in vitro toxicology. Together with guest speakers from ExxonMobil Biomedical Sciences and The Research Institute for Fragrance Materials (RIFM), we presented the latest GARD® data on quantitative risk assessment and regulatory testing of skin sensitizers, and the potential to further broaden the GARD® applicability. |
|
|
 |
| Dr Allison Greminger of Exxon Biomedical Sciences presenting the use of the GARD®skin assay to detect dermal sensitization potential in UVCBs and formulated lubricant products. |
|
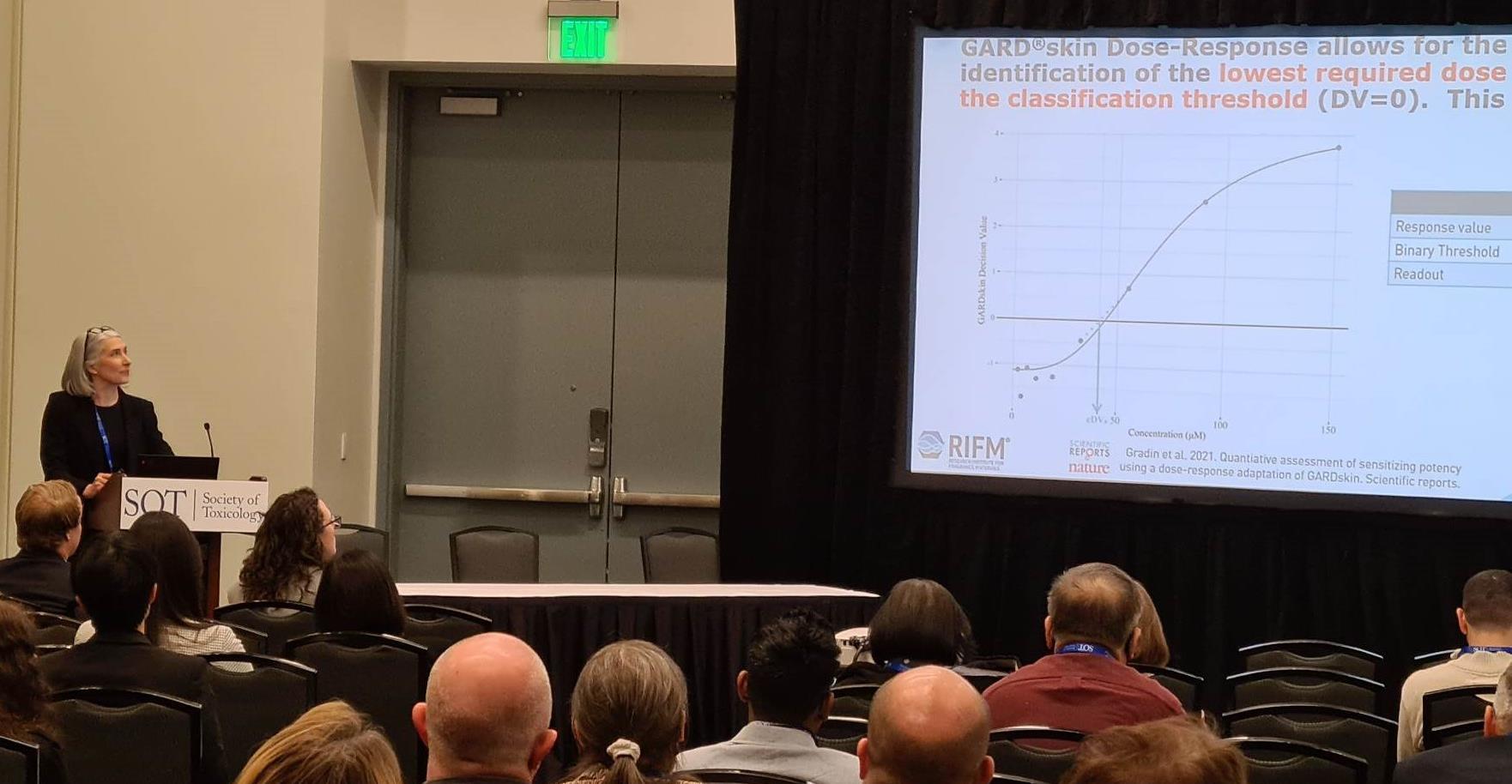 |
| Guest speaker Gretchen Ritacco presenting the potential to further broaden the applicability of the GARD® assay to assess the endpoint of photosensitization in RIFM's safety assessment program. |
|
|
|
 |
We are very pleased that yet another global chemicals company has chosen to collaborate with us, using GARD® skin to assess whether their new product candidates can cause allergic reactions on the skin.
Read more about the collaboration |
|
|
 |
Swedish Medtech company Duearity has successfully used results from in vitro assays for the endpoints of cytotoxicity, skin irritation and skin sensitization (GARD®skin Medical Device) to demonstrate the safety and performance of their medical device in their biological evaluation. Recently the company obtained CE marking under the MDR using the generated data. Duearity avoided unnecessary testing on animals and saved time to market.
Please provide me with the case study on this project |
|
|
 |
| In case you missed our Exhibitor-Hosted session at the SOT2023 Annual Meeting, we are hosting a free recap webinar on April 26th. Read more and register by using the button below. |
|
|
|
|